Empowering the world with transformative technologies to fight against infectious disease
Covid-19 Response
Addressing the unmet medical needs of patients with a serious or life-threatening disease or condition is important to BOA Biomedical. BOA Biomedical does maintain an Expanded Access program for circumstances where a patient with a life-threatening illness, such as COVID-19, requires treatment and the treating physician believes that the GARNET technology may be of benefit.

Compassionate Use
With GARNET™
The Compassionate Use provision in the regulations provides a path to access investigational products that have not yet received FDA approval or clearance, including the GARNET™ extracorporeal therapeutic that is intended to treat serious and/or life-threatening diseases.
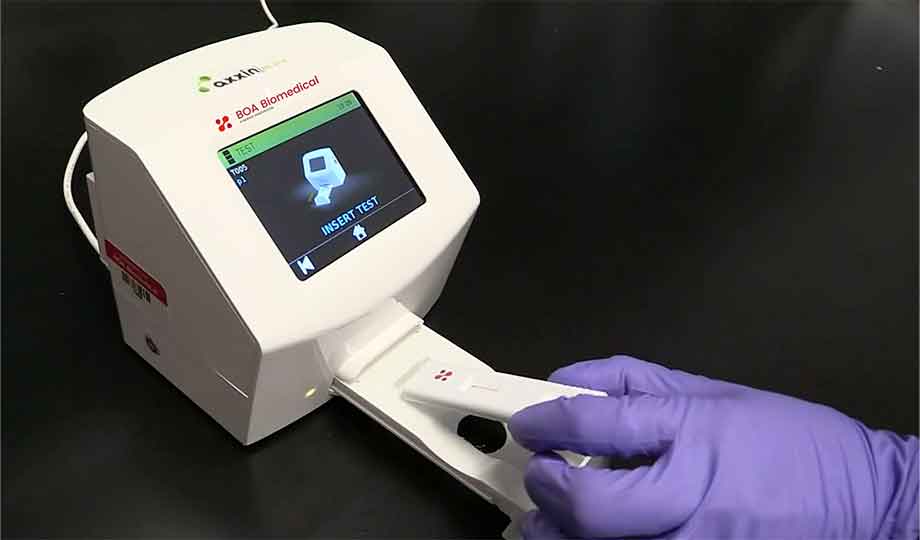
Rapid POC Infection/Sepsis Diagnostic
Rubellite™
BOA’s rapid pan-pathogen detection system recognizes active infection caused by bacteria, fungi, viruses and parasites directly from a patient’s sample within minutes


Rapid Pathogen Identification
Amethyst™
BOA’s culture-free, rapid pan-pathogen identification system determines which pathogen(s) are responsible for an active infection directly from a patient’s blood sample in less than one hour.
Pathogen Removal from the Bloodstream
Garnet™
BOA’s extracorporeal therapeutic filters pathogens and their by-products directly from a patient’s blood. (Placeholder Video)

Harvard University
WYSS INSTITUTE
BOA Biomedical, a Miraki Innovation company has licensed technology from Harvard’s Wyss Institute to develop devices that can diagnose and treat infectious diseases.
Department of Defense
diagnosing and treating sepsis
The U.S. DOD and their research agency, DARPA, committed $150 million to research focused on diagnosing and treating sepsis. BOA licensed the technology from Harvard University in 2018 and received ~$46 million of Emergency Grants from the U.S. DOD in 2020.